Where did the water go? II
| Y4, 6, 8, 10 (07/2007) | |||
| a) | i) | Simon's |
easy (Y4) very easy (Y6, 8, and 10) |
| ii) |
Any one of:
|
Refer to Working with Students for further discussion | |
| iii) |
reference to barrier, e.g., The lid stopped it getting out. |
easy (Y6, 8, and 10) Refer to Working with Students for further discussion |
|
Teacher background
Why is evaporation an important concept for students to know?
Water is essential for the existence of life. It impacts on our lives positively, but can also have negative impacts. Water in its various states has implications for our health (for example, managing condensation in houses), our property (for example, floods), our economy (for example, water use), and wider environmental issues (for example, climate change). To manage this resource we need to understand how water changes, and what drives these changes.
Key competencies and the Nature of science

At Level 2, Communicating in science includes attention to science vocabulary. The context of changes of state incorporates words that are very precise in their meaning. If students are to communicate their science ideas accurately, they need to learn these words. Go to Next steps,
Particle model
Particle theory describes all matter as consisting of very small particles (e.g., atoms, molecules) in a continual state of motion. The degree of motion is determined by how much energy they have and their relationship to other particles. The particle model helps us create mental models of what is happening inside the material at a level that is too small for us to see. It is useful for explaining at a micro level, using symbols, what we can actually observe in changes in state of a material, e.g. ice (a solid), liquid water, and water vapour (a gas).
Evaporation is a difficult concept for Level 2 students because:
- gas is invisible and so it is conceptually difficult for them to visualise; and
- the process of evaporation is also an abstract idea. Students can observe the results (water disappears/ can no longer be seen) but not how this happens.
At Level 2 the expectation is that most students' understanding will be limited to what they are able to see. (Particle theory is introduced at Levl 4.)
This resource was trialled with 655 students from Years 4-10, so the data collected provides a good indication of how students' ideas change over time. The trial numbers were Y4 (99 students), Y5 (15 students), Y6 (115 students), Y7 (51students), Y8 (128 students), Y9 (64 students) and Y10 (183 students).
NOTE 1: In this section the data from the separate years is considered. However, Year 5 was a particularly small group of just 15 students from one school who performed better on some questions than older students, probably indicating a school effect. Their results are not shown on the graphs.
NOTE 2: The results from the smaller samples of trial students will have a greater margin of error than the larger samples.
Students' responses to the questions uncovered the same misconceptions and partially correct responses that have been identified in international research (Salient points from the literature about understanding the water cycle). However, as you would expect, the number of students holding these misconceptions generally decreased with age. The information in the following graphs compares responses of the trial students in different year cohorts.
Question a) ii)
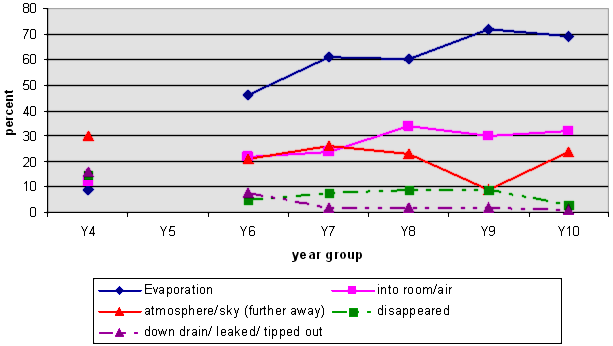
NOTE 2: There is no line linking the Y4 responses to the others because we did not include Y5 data.
NOTE 3: Some students' responses included more than one category. For example, they may have used the term "evaporation" and identified the sky as where the water went.
The graph shows that at Year 4 the most likely response was that the water went somewhere distant, such as the sky. This is a first step to understanding the concept of evaporation, although very few used that term. The two next most common responses were that it had just "disappeared", or no longer existed, or that it had been tipped out or the jar had leaked.
There was a marked increase between Years 4 and 6 in students being able to talk about evaporation and/or a change of state to explain the disappearance. The graph also shows that, as the students get older, the percentage of naïve explanations decreases, although they don't entirely disappear.
The following common misconceptions or partial explanations were noted:
It goes into the atmosphere/clouds/sky/sun (distant location)
While some of these responses may not be incorrect, e.g. "the sky", many in this category were indicative of common misconceptions. Students giving this response often use the word "up" and only think of the typical water cycle pictures where the evaporated water gathers to form clouds, and then falls as rain, hail, or snow.
Some of the students talked about the water being "sucked up" by the sun, or "going into" the clouds. This was more common with, though not confined to, younger students.
Dried up/ nowhere/ disappeared
There were few students who gave this type of response. However, there were still examples at all years.
Down the drain/ tipped over/ tipped out/ leaked
This was not a common response, and by Year 7 very few students thought that the water had gone elsewhere as liquid water. An occasional student suggested that Tom might have taken Simon's water, or that Tom's might have leaked.
There were some examples of students using scientific terms incorrectly.
dissolved by the sun (Year 4)
dissolved into gas (Year 4)
The sun melted it so it escaped (Year 4)
Condensation melts back into water (Year 9)
a) iii)
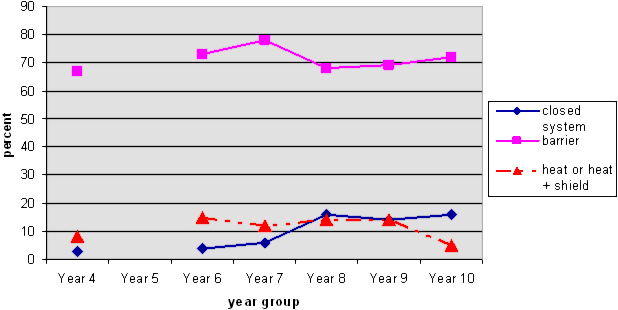
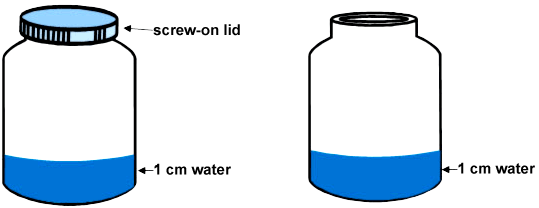
Student responses: Explain why the other jar still had water in it?
NOTE 1: The solid lines represent acceptable answers. The dotted lines represent misconceptions.
NOTE 2: There is no line linking the Y4 responses to the others because we did not include Y5 data.
This graph shows that at all years about 70% of students identified that the water couldn't get out because of the lid. This is known to students because of their observations in everyday situations.
Very few students at any age described Tom's jar as a closed system, i.e., the water that evaporated then condensed on the lid and dripped down again (only 3 Year 4 students hinted at this idea). Not all of these students mentioned a barrier, but would have been aware that there was one.
Students who mentioned heat (again, very few Year 4 students) know that there is a link between heat and evaporation, but they are still coming to grips with this idea. They may think that evaporation does not occur when it is cold (rather than a factor that speeds up evaporation), or they may be focusing on an irrelevant factor for the question (temperature rather than the barrier). Some thought that heat could not "get in" the covered jar, while others thought that the heat could not "get out". The graph suggests that this is more likely to happen at Years 6-8, after which most students realise it is irrelevant to this question.
Evaporation
Evaporation is a difficult concept for students. There are two things they need to understand before they can really create a mental model of evaporation:
- Water can be a gas (difficult because it is invisible); and
- Water is made up of tiny particles (i.e. the particle nature of matter). At Level 2 most students will not be ready for particle theory, but they can discuss notions of something not being visible, but still there in reality.
To begin developing concepts of evaporation students need to be exposed to many examples of its occurrence in every day situations – this is especially important at Level 2. Some examples of contexts that students are likely to be familiar with are puddles drying up after rain, drying clothes, dishes drying on the bench, sweating, drying after a swim, and pots boiling dry. Discuss their observations and share their theories with others about what could be happening. Students will often retain their naïve explanations, e.g., "the sun sucks up the water", or "it leaked out" despite your best efforts. However, understanding a complex concept such as evaporation is a bit like doing a jigsaw puzzle. Once students have enough of the pieces they will begin to see how they fit together.
Role play can provide a more concrete experience of an abstract idea. After observations, ask students to act out the invisible water leaving the jar, pot, or puddle.
Explore condensation and evaporation together so it is easier to make sense of the disappearing/appearing water. Ask them to be creative, for example:
"Let's think of all the places the water may have gone",
and also critical:
"Is that very likely?"
"Can we think of examples of when water just seems to appear from nowhere?"
"What else have you noticed when this happens?"
"Let's think of all the places it might have come from."
Developing scientific vocabulary
Introduce scientific terms. Using the word "evaporation" does not necessarily mean that students understand the process. However, using the correct scientific vocabulary cues students into wearing their "science hats", and contributes to developing a precise vocabulary to describe their experiences.
The word "gas" often causes problems, because students are familiar with it in everyday terms of "putting gas (petrol) in the car". This is a good example of a word with a specific scientific meaning that is different to its everyday meaning. For more examples go to Language barriers.
Steam is often confused with water vapour, even by adults. We can see steam because some of the water vapour is condensing, so there is a mix of water vapour and liquid water. We can also see clouds, mist, and fog for the same reason. Some students may call these "smoke".
Self/peer assessment
Make up 2 sets of cards (see Other resources), one with the scientific terms that describe changes of state, the other with the definitions, which students, working in groups or independently, match. They can check their own or another group's answers against a marking sheet. This could also be done as a class activity where different groups hold a card and have to negotiate which group has the other half.
The cards could also be adapted to make a Loopy game. Loopy is modeled in Interdependence loopy.
Self/peer assessment cards for checking vocabulary
| Melt | Ice changes to liquid water |
| Freeze | Liquid water changes to ice |
| Evaporate | Liquid water changes to water vapour |
| Condense | Water vapour changes to liquid water |
| Ice | Frozen water |
| Liquid water | Water that can be poured |
| Water vapour | Water that is a gas (we cannot see it) |
| Steam | A mixture of water vapour and small drops of liquid water |
- Ministry of Education (1998). Making better sense of the material world. Wellington: Learning Media. See the section on water.
- Ministry of Education (2001). Building Science Concepts, Book 15. Where's the water? Wellington: Learning Media.
Level 2 ARB resources about evaporation:

