Patterns on the Periodic Table
This task is about recognising patterns on the periodic table.
Dmitri Mendeleev was a Russian chemist who is credited with the invention of the periodic table. [Learn more about Mendeleev here]
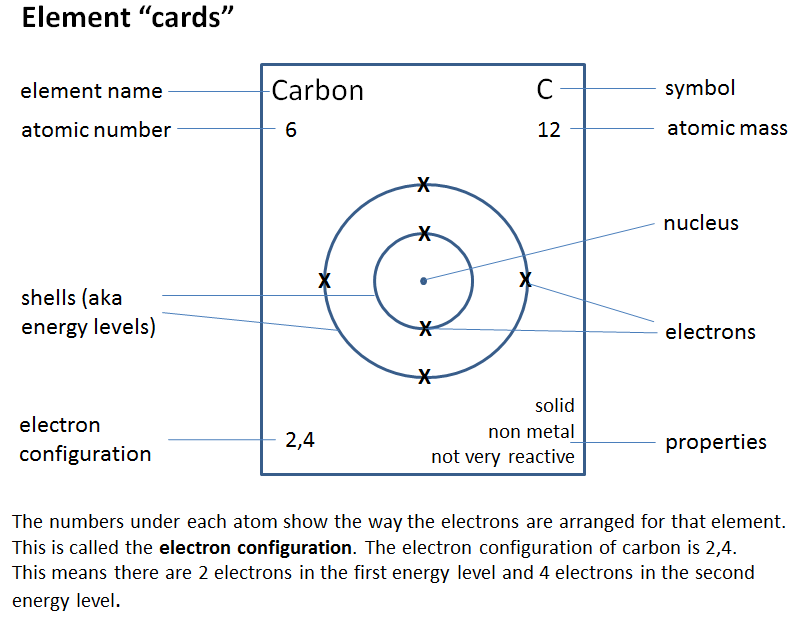
For this exercise you will be using information on element cards such as the one below to make conclusions about patterns in elements on the periodic table - very much like Mendeleev did back in the mid 1800s.