This task is about fair testing and reading information from a graph.
|
10 g of each of the following metals: copper, iron, magnesium, and zinc were polished, and then left to corrode on a shelf in a laboratory for 3 months. They were then polished again, the mass loss worked out, and the results graphed.
Mass loss of metals 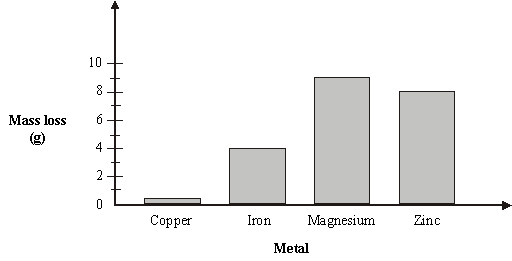 |
|
| a) |
Which metal corroded the most? ____________________
|
| b) |
How much iron corroded? ____________________ g
|
| c) |
How much zinc was left? ____________________ g
|
| d) |
What was the total mass of all the metals after 3 months? (Show your working).
__________ g.
|
| e) |
Write an aim for this experiment.
|
|
f) |
Why were the metals polished at the end of 3 months? |
|
g) |
Why was the starting mass of each metal the same? |

