Petrol combustion
This task is about complete and incomplete combustion.
Here are two equations describing the burning of petrol.
-
2C8H18 + 25O2
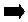 16CO2 + 18H2O 10808 kJ energy released
16CO2 + 18H2O 10808 kJ energy released -
2C8H18 + 14O2
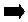 6C + 10CO + 18 H2O 7624 kJ energy released
6C + 10CO + 18 H2O 7624 kJ energy released

