Patterns on the Periodic Table
0
Overview
Using this Resource
Connecting to the Curriculum
Marking Student Responses
Working with Students
Further Resources
This task is about recognising patterns on the periodic table.
Dmitri Mendeleev was a Russian chemist who is credited with the invention of the periodic table. [Learn more about Mendeleev here]
For this exercise you will be using information on element cards such as the one below to make conclusions about patterns in elements on the periodic table - very much like Mendeleev did back in the mid 1800s.
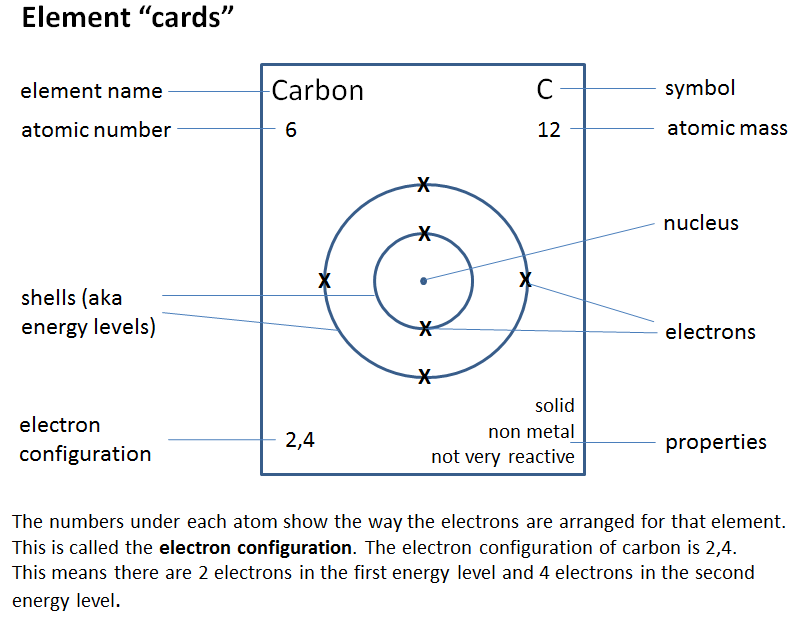
Task administration:
This task can be completed with pencil and paper and online with automarking.
Level:
5
Curriculum info:
Key Competencies:
Keywords:
Description of task:
In this task, students look for patterns in electron arrangement in the periodic table. They use their ideas to place missing elements in the table, answer some questions about the patterns, and use the patterns to predict the properties of some elements.
Curriculum Links:
Science capabilities
The capabilities focus is brought about by the conversations students have and the questions they ask.
Capability: Gather & interpret data
This resource provides opportunities for students to discuss the data/evidence scientists collect and the patterns they notice in order to answer a question about classification and/or identification.
Science capability: Gather and interpret data (TKI)
Capability: Interpreting representations
This resource provides opportunities to discuss how scientists use a model to convey information about things we cannot see. The periodic table and the structure of the atom is one “shorthand” way scientists do this.
Science capability: Interpreting representations (TKI)
Science capabilities:
Answers/responses:
| Student response | Y9/10 (03/2017) | |
|
a)
|
|
very easy
(all 6 correct)
|
| b) |
i) As you go down each column (group) of the periodic table the number of electrons in the outer shell stays the same and the number of shells increases.
ii) What is calcium’s atomic number? 20
iii) What is Calcium’s electron configuration? 2,8,8,2
iv) I predict Calcium will have the following properties: It will be solid at room temperature, it will be a metal and it will be reactive.
|
easy
easy
easy
moderate
|
|
c)
|
i) As you go across each row of the periodic table the number of electrons in the outer shell increases and the number of shells stays the same.
ii) The atomic number of Nitrogen is 7
iii) Atomic mass of Boron is: 10.5 (accept 10 or 11)
iv) The electron configuration for Nitrogen is 2,5
|
easy
easy
moderate
easy
|
Diagnostic and formative information:
|
Question
|
Common error | Likely reason | Next steps |
|
b) i)
c) i)
|
Students incorrectly saying the number of electrons in the outer shell increases down the column (group) of the periodic table.
|
Students didn't recognise that the number of electrons in the outer shell is the same for all of the elements in a column (group). Some may have looked at the total number of electrons (atomic number), or misinterpreted the question, not noticing the words 'in the outer shell'.
|
Provide further opportunities for students to explore patterns recognition when looking at tables and diagrams.
Create practical activities which reinforce observation skills, e.g., making their own drawings or making models, etc.
|
|
b) ii)
iii)
c) ii)
iii)
iv)
|
Students confused the atomic mass, atomic number, and the electron configuration, e.g.:
|
Unfamilarity with the terms and/or not using the supporting question stimulus material effectively. Students did not know the atomic number equals number of electrons in an atom or the electron configuration is the way the electrons are placed in shells around the nucleus, e.g., 2,8,8,2
|
Provide other activities that define and use the terms atomic number, atomic mass, and electron configuration. |
| b) iv) | About half of students couldn't predict all 3 properties of calcium correctly. | Students who were unable to answer this question did not recognise the patterns in element properties in the information given on the element cards. They also may not have made the connection between the patterns observed in electron arrangement and the patterns observed in element properties. | Read about and discuss how Mendeleev grouped the elements in the way he did (see the link in the question stimulus material). Discuss how the number and arrangement of electrons could influence the properties of the elements, e.g., why it's no accident that the metals are all on the left hand side of the periodic table and non metals are on the right hand side.This could lead to a more general discussion about the importance of thorough and systematic observation in scientists' work. |
Based on responses from 120 Year 9 and 10 students.
Next steps:
Capability 1: Gather and interpret data
Paying attention to features is important in science to support how things are grouped/classified. An important aspect is to look at similarities and differences.To help students to notice accurately give them opportunities to look for similarities and differences. A good strategy is to share their work, so they build on others' ideas. Things they could look for:
- does the evidence/data given support the explanation/conclusion?
- is there any data missing?
- what are the differences/similarities?
- is the evidence/data sufficient to support the explanation/conclusion?
Capability 4: Interpreting representations
Scientists represent their ideas in a variety of ways, including models, graphs, charts, diagrams and written texts. Students need to know quite a lot about science knowledge to be able to interpret/understand all the information a model is telling us. To support students to test their understandings of representations/models, give them opportunities to compare/review the periodic table. Get them to ask:
- what is being represented?
- how is each representation different?
- is there a pattern/patterns in the representations?

