What is happening II?

| Y4, 6, 8, 10 (07/07) | |||
| a) |
i)
ii)
|
Melting/turning into water
Either, or combination of:
|
very easy (Y4, Y8, & Y10)
easy (Y6)
very difficult (Y4 and Y6)
easy (Y 8 and Y10)
|
| b) | The air (in the room) |
very difficult (Y4, Y6, & Y8) difficult (Y10) |
|
Results are based on a trial set of 655 students from Years 4-10, in July 2007. The difficulty levels are based on student data from Year 4 (99 students), Year 6 (115 students), Year 8 (128 students), and Year 10 (183 students).
Why is "changes in state of water" an important concept for students to know?
Water is essential for the existence of life. It impacts on our lives positively, but can also have negative impacts. Water in its various states has implications for our health (for example, managing condensation in houses), our property (for example, floods), our economy (for example, water use), and wider environmental issues (for example, climate change). To manage this resource we need to understand how water changes, and what drives these changes.
Particle theory
Particle theory describes all matter as consisting of very small particles (e.g., atoms, molecules) in a continual state of motion. The degree of motion is determined by how much energy they have and their relationship to other particles. The particle model helps us create mental models of what is happening inside the material at a level that is too small for us to see. It is useful for explaining at a micro level, using symbols, what we can actually observe in different states of a material, e.g., ice (a solid), liquid water, and water vapour (a gas).
This resource was trialled with 655 students from Years 4-10, so the data collected provide a good indication of how students' ideas change over time. The trial numbers were Y4 (99 students), Y5 (15 students), Y6 (115 students), Y7 (51students), Y8 (128 students), Y9 (64 students) and Y10 (183 students)
NOTE 1: In this section the data from the separate years are considered. However, Year 5 was a particularly small group of just 15 students from one school who performed better on some questions than older students, probably indicating a school effect. Their results are not shown.
NOTE 2: The results from the smaller samples of trial students will have a greater margin of error than the larger samples.
QUESTION A
a) i) What is happening to the ice cubes?
While most students at all years answered correctly that the ice was melting, there were also some other responses that were consistently given across all years by just a few students.
"Getting smaller"
While not incorrect, there is no evidence of understanding that a change of state has occurred. However, the observation provides a good starting point for further exploration of what happens to the ice, and the introduction of vocabulary (melting).
"Cooling the water"
The focus of this answer is on the water rather than the ice, and what the ice is causing to happen, rather than what is happening to the ice. It does indicate that there is an awareness of heat transfer. Follow up questions to ask:
- What is happening to the ice (as the water cools)?
- Where has the (heat) energy come from to melt the ice?
- When all the ice has melted will the water be warmer or cooler than it was at the beginning?
Responses that do not involve a change of state (e.g., floating, sinking, sticking together, changing colour, cracking)
These answers are focussed on what the ice is doing (what the student can observe), rather than what is happening to it.
Incorrect use of scientific terms e.g., dissolving
Students at Years 6 and 8 were most likely to use the wrong terms.
a) ii) Why is this happening?
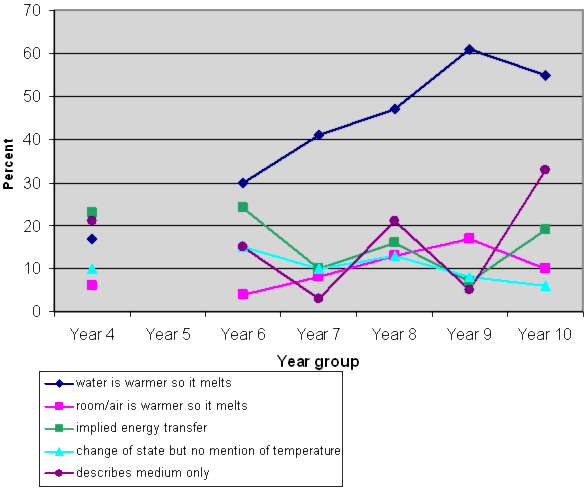
Student responses: Why is this happening?
NOTE 1: There is no line linking the Y4 responses to the others because we did not include Y5 data.
NOTE 2: Some students' responses included more than one category. For example, they may have identified both the water and the surrounding air as factors.
The water temperature is the most important factor. However, room temperature is also a factor, both directly to the ice above the water, and indirectly by affecting the water temperature. Predictably, more students identified the water temperature than the air temperature. However, by Year 10 there were still less than 2/3 of students answering this question fully, i.e., linking temperature of the medium (the water or air) to a change of state. Some examples of student responses that did make this link are:
- The water is too warm so it just malts (Year 4)
- The water isn't as cold as the original place where it has been frozen (Year 6)
- Because the water surrounding them is warmer than they are (Year 8)
- Because the water is a higher temperature than the ice (Year 10)
Implied energy transfer:
- Because the sun is shining at the glass (Year 4)
- Because it is too warm (Year 6)
- The warmth is slowly melting it (Year 8)
- Because of a change of temperature (Year 8)
- Warmer environment (Year 10)
- Because the water is taking the ice so it will turn into water (Year 4)
- Because it is made out of water (Year 6)
- Because they have come in contact with a liquid therefore slowly turning into a liquid itself (Year 8)
Just mentions medium
- Because of the water (Year 6)
- Because the water is making the ice cubes melt (Year 10)
Identifies time without relating to conditions
- Because the ice cubes were in the water for long (Year 6)
- Because it has been in there for too long (Year 6)
- i) they are sinking
- ii) because of the force of the person putting it in.
A few students at all years used science vocabulary inappropriately, for example:
- Because it is dissolving (Year 4)
- Because if you put ice in it would melt because all of the lickworlds would dezolve it (Year 6)
- The heat is evaporating the ice into the water (Year 10)
- Because the water in the glass is dissolving the ice cubes (slowly) (Year 10)
There are tiny droplets of water forming on the outside of the glass. Where is this water coming from?
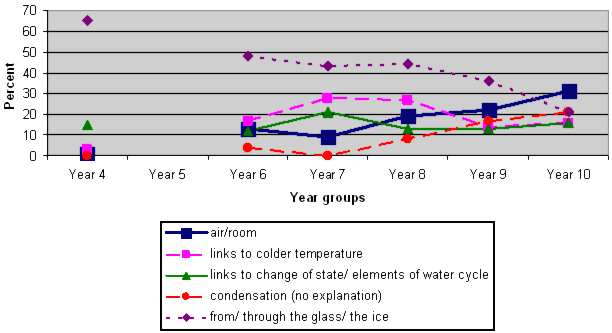
Student responses: Where is the water coming from?
NOTE 1: There is no line linking the Y4 responses to the others because we did not include Y5 data.
NOTE 2: Some students' responses included more than one category. For example, they may have made links to both the water cycle and condensation. The thick blue line represents the scientifically acceptable answer – from the air, or from the room. Only 1 Year 4 trial student gave this response, and even at Year 10 only about 1/3 showed clearly that they understood this.
Some students further explained that the water came from the air (shown by the continuous green line), although not always mentioning air, by describing changes of state; for example:
The water that has heated up and turned into steam (Year 4)
It's evaporating from the glass and when it lands on the cold glass again it turns into liquid again (Year 9)
The pink and red broken lines on the graph represent responses that may indicate a partial understanding, or possibly a misconception. These are described in the chart below.
| Partial understanding | Examples of student responses | Comment |
|
Condensation (without any further explanation) |
Very few Year 4 students used the term at all. Older students were most likely to say the water on the outside of the glass was condensation, without any further explanation. |
Using the correct scientific term indicates that students have at least met the concept, although it does not, by itself, demonstrate that they have an understanding of it. |
|
Links to colder temperature
|
From the water vapour that's cooling down (Year 9) All of the coldness makes little drips of water on the outside. (Year 6) The cold water on the glass makes it cold on the outside of the glass and the coldness of the outside forms droplets of water (Year 8) |
The observation that condensation appears on a surface colder than the temperature of the air in which the water vapour is present is a beginning step in understanding the role of temperature in changes of state. |
A misconception is shown by the purple dotted line.
| Misconception | Examples of student responses | Comment |
| The water came through the glass. |
It is coming from the water in the cup (Year 6) |
This is a common misconception that was prevalent at Year 4 and decreased as students got older. However, at Year 10 about 20% of the trial students still held this misconception. |
|
The water came from the ice. |
The ice cubes (Year 4) |
A number of students from all years focussed on the ice. Although we grouped these answers with the "through the glass" responses, students may actually have been referring to the role of the coldness of the ice. Asking students to explain why they gave their answer will give a better indication of their thinking. |
A few students (too few to show on the graph) thought that the water came from somewhere else. This response is also reported in research about students' understanding of condensation.
- It is coming from when you drink it (Year 4)
- When the ice went in it probably splashed some drops from the outside (Year 6)
Students' responses to the questions uncovered the same misconceptions and partially correct responses that have been identified in international research. However, as you would expect, the number of students holding these misconceptions generally decreased with age. To find out more about common misconceptions identified by research go to Salient points from the literature about understanding the water cycle.
Next steps
There are three science ideas that students need to understand before they can really create a mental model of condensation:
- Water can be an invisible gas (called water vapour);
- Water is made up of tiny particles (particle theory); and
- Water vapour changes to liquid water when it comes into contact with a cold surface.
All of these are difficult ideas because of the invisibility of water vapour. To begin developing concepts of condensation students need first to be exposed to many examples of its occurrence in every day situations. Some examples of contexts that students are likely to be familiar with are:
- Running windows and walls after having a bath or shower, or when clothes are drying:
- Water on windows on cold nights;
- Dew on grass, and
- Breathing on windows.
Understanding that water can exist as a gas
Explore condensation and evaporation together so it is easier to make sense of the disappearing/appearing water. It also helps them see that condensation and evaporation are reverse processes. Students who have noticed that temperature is one factor can also explore the idea that energy is added and energy is taken away in these two processes. (The energy may not always be heat energy. For example, wind will result in faster evaporation. Wind energy is transferred in the form of heat to the water droplets, which move about and slowly break away as water vapour.)
Ask students to be creative, for example:
- "Let's think of all the places the water may have come from",
- "Is that very likely?"
- "Can we think of examples of when water just seems to appear from nowhere?"
- "What else have you noticed when this happens?"
- "Let's think of all the places it might have come from."
Encourage them to consider the idea that although we cannot see the water it is still there, but in an invisible form.
Provide opportunities for students to test their ideas, using their responses to develop an investigative question, and then plan and carry out fair tests. For example, those students who think the water comes through the glass could compare what happens to a glass containing just water with one that also contains ice.
Understanding that water is made up of tiny particles/ molecules
Role play can provide a more concrete experience of an abstract idea. Provide scenarios of water appearing and disappearing, ask students to act out the water as it changes state. At all levels there should be a focus on developing the idea that, even though we cannot see the water sometimes, it is still there.
- With very young students they may be just acting out disappearing and reappearing water.
- Students may then "be" water molecules, becoming more energetic as they get warmer and moving further apart as they turn to water vapour; becoming less energetic as they cool and moving closer together as they turn into liquid water.
- Older students might work in threes to demonstrate that a water molecule is made up of two hydrogen atoms and one oxygen atom. An important teaching point is that the three stick together no matter what state the water is in.
Role play can also be a useful strategy for assessing students' understanding of particle theory. Students could work in groups to act out a provided scenario. If you want to include peer assessment, develop as a class some judgement criteria suitable for the level of their understanding, and then ask them to provide feedback about each group's performance. Alternatively, the actors could be asked questions about the significance of elements of their performance.
Understanding the role of temperature
Encourage students to generalise from one context to another (see lists of contexts above). For example, compare the temperature inside and outside a room when condensation forms on windows, then compare this with breath on a mirror.
Developing science vocabulary
Help students develop a specific definition of condensation in language relevant to their age and level of understanding. In our trials we found that students were less likely to use the term "condensation" than "evaporation". When it was used, students often did not give an indication that they understood the process. The SPACE research report (Russell & Watt, 1990) noted that in everyday conversation condensation is limited to the water that appears on the inside of windows, whereas in science the term refers to the process. See also Language of science (Specialised language).
ARB resources:
For ARB resources that assess the concept of condensation, click on the link or use the keyword. Condensation. Then sort by level to find resources at the level you require.
If you also want to address the role of condensation in the water cycle click on the link or use the keywords condensation OR water cycle.
For further information about students' ideas about condensation go to Salient points from the literature about understanding the water cycle.
An English resource, Changes of state II, is about finding the features of a science test. The context is changes of state.
Resources in schools:
- Ministry of Education (2003). Building Science Concepts, Book 31. Water and weather. Wellington: Learning Media.
- Ministry of Education (2001). Building Science Concepts, Book 15. Where's the water? Wellington: Learning Media.
- Ministry of Education (1998). Making better sense of the material world. Wellington: Learning Media.
The following Connected journals include useful articles.
Ministry of Education (2004). Connected 2. Wellington: Learning Media.
Hard ice, soft ice.
Ministry of Education (1999). Connected 2. Wellington: Learning Media.
Strange white world / Mist
Exemplar:
Water and Ice – This Level 1 exemplar is about ice melting in the sun.
Research report for teachers:
Russell, T. and Watt, D. (1990). Evaporation and Condensation. Liverpool: Liverpool University Press.

