Acid, alkali or neutral
This task is about identifying the effect of indicators on acid, alkali and neutral substances.
|
Part A: News from hues
|
|
| 1. | Put a drop of red cabbage indicator solution in each spot in the top row of a spotting tile. |
| 2. | Cut the red litmus paper into four pieces, place a piece in each spot of the middle row of the spotting tile. |
| 3. | Cut the blue litmus paper into four pieces, place a piece in each spot of the bottom row of the spotting tile. |
 |
|
| 4. | Add one drop of white vinegar to each spot in column 1. |
| 5. | Add one drop of baking soda solution to each spot in column 2. |
| 6. | Add one drop of water to each spot in column 3. |
| 7. | Add nothing to each spot in column 4. |
| 8. | Write your results in the circles above. |
|
Results
|
|
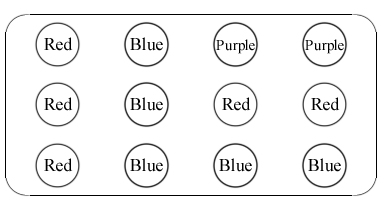
| a) |
Use coloured pencils to colour in the spots on the table above to show the colour changes that have occurred.
NOTE: white vinegar is an example of a weak acid, baking soda solution is a weak alkali, and water is a neutral solution.
|
| b) |
Complete these sentences
Acids make indicators go ______________________.
Alkalis make indicators go _______________________.
Neutral solutions have _______________________ on the colour of a substance. |
| c) |
Why did one column of the spotting tile have nothing added to it?
|
Part B: Acid, alkali or neutral
|
||||||||||||||||||||||
| a) |
Complete this table to show your results.
|
|||||||||||||||||||||
|
b)
|
Rank the substances you tested from the strongest acid to the strongest alkali.
|
|||||||||||||||||||||


 orange drink
orange drink