Acids and bases
| Y10 (08/2009) | |||
| a) |
i)
ii)
iii)
|
5
9
13
|
very easy
very easy
very easy
|
| b) |
i)
ii)
iii)
|
True
False
True
|
very easy
very easy
very easy
|
| c) | C | difficult | |
Why knowing about pH is importantUnderstanding the preference of pH levels for different organisms can increase productivity. For example in fish farming it is important to know that saltwater fish prefer water at a basic pH of 8.0 or above and freshwater fish thrive in a range between 5.5 and 7.5 depending on the specific species. Fruit and vegetable growers know that tomatoes prefer a soil with a pH level between 5.5 and 7.5 whereas cabbages thrive best in soils between pH level 6.0 and 7.5. To increase soil pH gardeners typically add a base such as lime. To reduce soil pH they typically add an acidic substance such as sulphur or decayed vegetable matter, compost, urea, etc.
| Inaccurate responses | Likely misconception | |
| a) | Answering solution A or B etc instead of the pH level of these solutions on the scale. | Some students did not connect / read the two pieces of information (scale and written list) to answer the question. |
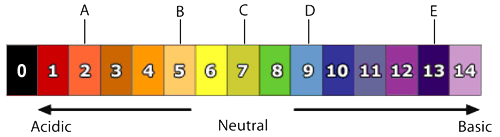
| b) ii) | Reading pH 13 as an acid. | Some students did not separate the scale into an acid and a basic scale beginning in the middle at pH 7. Instead they read the whole scale from 1 to 14 as an acidic scale. |
| b) iii) | Reading neutral as 8 or 6. | Some students may not have been aware that neutral meant the middle of the scale at pH 7. |
| c) | Choosing pH 13. | Students may not have been aware that the pH scale is logarithmic and a slightly basic soil would be just over neutral e.g. 7.2 through to 7.9. |
| Students chose three options pH 7, pH 9 and pH 13. |
Students may not have been aware that when mixing the same amount of a base and an acid the resultant pH level is between the two, e.g.,pH 13 (strong base) and pH 5.5 (weak acid) = pH of a weak basepH 9 (base) and pH 5.5 (weak acid) = pH slight/very weak basepH 6.5 (slight/very weak acid) and pH 5.5 (weak acid) = pH weak acid |
|
| Choosing pH 7. | Students who chose pH 7 may not have been aware that water dilutes the acid but does not increase the alkalinity. Only a base added to an acid or an acid added to a base can result in a neutral solution. | |
| Choosing the citrus peel at pH 6.5. | Students could have been distracted by prior knowledge that vegetable compost is used on soils. |
Because students have difficulty understanding the range across the pH scales and why pH strength changes, they could do the following activities:Understand about logarithmic scalesA logarithmic scale is one where each number on the scale increases ten fold. As the pH scale is logarithmic each whole pH value below 7 is ten times more acidic than the next higher value. For example pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. It is the same for values above pH 7 where pH 10 is ten times more basic than pH 9 and 100 times (10 times 10) more basic than pH 8. Therefore to understand the amount of solution (logarithmic scale) needed to change a pH level e.g. 4 to 5 students could:
- titrate a base into an acid and note how much base was needed to change the universal indicator to the next colour;
- explore other logarithmic scales, e.g. Richter scale for measuring earthquakes.
Practise changing pH levels by:
- mixing the same amounts of different strength acids and bases and interpreting the strength of the resulting product by reading the indicator’s colour change, e.g., a strong acid with a weak base should produce a moderately strong acid (universal indicator colour change red to orange);
- practising adding a base to an acid to reach a neutral solution.
Understand pH and hydrogen levelsStudents could learn that it is the amount of dissolved hydrogen ions (H+) that affects the level of acidity of a solution. pH describes the number of positive hydrogen ions H+ and as the number of H+ increases the number of OH– (hydroxide ions) decreases. So
- acidic is when there are more H+ (hydrogen ions) than OH– (hydroxide ions).
- neutral is when the H+ (hydrogen ions) are equal to the OH– (hydroxide ions).
- basic is when there are fewer H+ (hydrogen ions) than OH– (hydroxide ions).
Because students have difficulty understanding the effect of water on the pH levels of a solution they could do the following activities:
Understanding about neutralisation Discuss that, although water is described as a neutral substance, it does not neutralise a solution. Adding water is effectively just diluting the solution, a physical change, whereas neutralisation is a chemical reaction between hydrogen ions and hydroxide ions to produce water. Only a base added to an acid or an acid added to a base can result in a neutral solution.
Understanding about dilutionDiscuss that if water is added to an acid the acid will become dilute as there is a lower percentage of acid in the aqueous solution e.g. the same number of H+ spread out in a more aqueous solution. Therefore the pH of the acid will increase (e.g., pH 3 to pH 6) to a very weak acid. Similarly a dilute base occurs when water is added so that there is a low percentage of the base in the aqueous solution, e.g., the same number of OH– spread out in a more aqueous solution. Therefore the pH of the base will decrease (e.g., pH 11 to pH 8) to a very weak base.
Students can investigate this by adding water to an acid solution and observing that the indicator colour does not change to a base colour and vice versa for a base to an acid. It should change to the colour of a dilute (weaker) acid/base.

Note: Adding water to an acid solution can result in splattering. Therefore when mixing acid and water, always add the acid to the water, and add it slowly. Never add the water to the acid.

