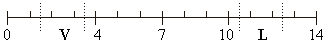Recording results of a pH test
This task is about using indicators to find the pH of four different liquids.
|
Susie wanted to find out what happens when the indicator solutions of
were added in turn to each of
|
|
| a) |
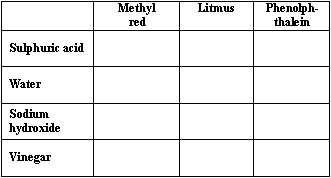
Draw a table that Susie could use to record her results. (You do not need to show any results).
|
| b) |
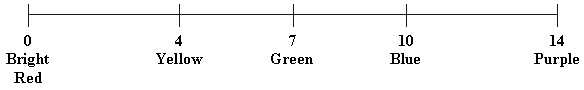
Below is the pH scale.
|
 |
|
|
When tested, vinegar turned the indicator orange, while lime water turned it dark blue.
Draw a V for vinegar and a L for lime water to show where these solutions would be on the pH scale.
|
|