Where did the water go?
| Y4, 6, 8, 10 (07/2007) | |||
| a) | i) | Simon's |
easy (Y4) very easy (Y6, 8, and 10) |
| ii) |
Any one of:
|
Refer to Working with Students for further discussion | |
| iii) |
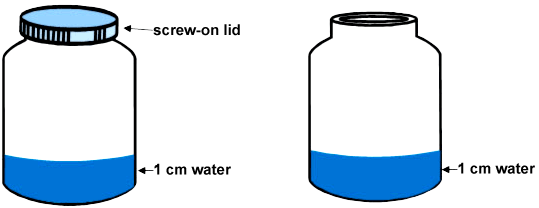
reference to a closed system, e.g., The lid prevented the evaporated water from escaping, so when it condensed on the lid it dripped back into the water in the jar. or reference to barrier, e.g., The lid stopped it getting out. |
Refer to Working with Students for further discussion | |
| b) | i) | yes |
very difficult (Y6) moderate (Y8 and 10) |
| ii) | Discusses evaporation and/or condensation, and/or identifies that the evaporated water cannot escape. | very difficult (Y6, 8, and 10) | |
| c) | i) | Tom's |
very difficult (Y6) moderate (Y8 and 10) |
| ii) |
Answers could include the following elements:
|
Refer to Working with Students for further discussion | |
Teacher background
Why is evaporation an important concept for students to know?
Water is essential for the existence of life. It impacts on our lives positively, but can also have negative impacts. Water in its various states has implications for our health (for example, managing condensation in houses), our property (for example, floods), our economy (for example, water use), and wider environmental issues (for example, climate change). To manage this resource we need to understand how water changes, and what drives these changes.
Particle model
Particle theory describes all matter as consisting of very small particles (e.g., atoms, molecules) in a continual state of motion. The degree of motion is determined by how much energy they have and their relationship to other particles. The particle model helps us create mental models of what is happening inside the material at a level that is too small for us to see. It is useful for explaining at a micro level, using symbols, what we can actually observe in changes in state of a material, e.g. ice (a solid), liquid water, and water vapour (a gas).
This resource was trialled with 655 students from Years 4-10, so the data collected provides a good indication of how students' ideas change over time. The trial numbers were Y4 (99 students), Y5 (15 students), Y6 (115 students), Y7 (51students), Y8 (128 students), Y9 (64 students) and Y10 (183 students)
NOTE 1: Year 4 students only completed Question a).
NOTE 2: In this section the data from the separate years is considered. However, Year 5 was a particularly small group of just 15 students from 1 school who performed better on some questions than older students, probably indicating a school effect. Their results are not shown on the graphs.
NOTE 3: The results from the smaller samples of trial students will have a greater margin of error than the larger samples.
Students' responses to the questions uncovered the same misconceptions and partially correct responses that have been identified in international research (Salient points from the literature about understanding the water cycle). However, as you would expect, the number of students holding these misconceptions generally lessened with age. The information in the following graphs compares responses of the trial students in different Year cohorts.
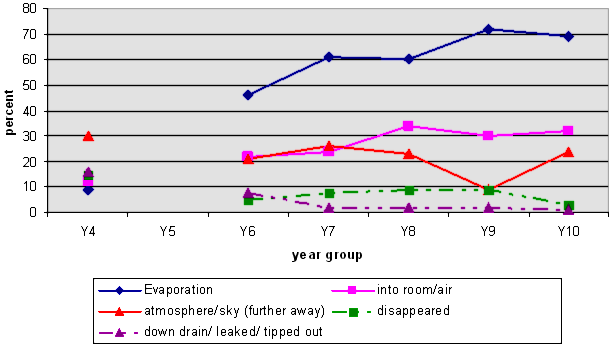
NOTE 2: There is no line linking the Y4 responses to the others because we did not include Y5 data.
NOTE 3: Some students' responses included more than one category. For example, they may have used the term "evaporation" and identified the sky as where the water went.
The graph shows that there was a marked increase between Years 4 and 6 in students being able to talk about evaporation and/or a change of state to explain the disappearance. There was a steady increase in students identifying that the water went into the air, until Year 8, when the number stays about the same. This may be because students are more likely as they get older to talk about the process of evaporation rather than the water's physical presence.
The graph also shows that, as the students get older, the percentage of naïve explanations decreases.
The following common misconceptions or partial explanations were noted:
It goes into the atmosphere/clouds/sky/sun (distant location)
While some of these responses may not be incorrect, e.g. "the sky", many in this category were indicative of common misconceptions. Students giving this response often use the word "up" and only think of the typical water cycle pictures where the evaporated water gathers to form clouds, and then falls as rain, hail, or snow. They may not be aware that an evaporation/condensation cycle can occur in a room, for example, or indeed in the closed jar (refer to Question b).
Some of the students talked about the water being "sucked up" by the sun, or "going into" the clouds.
Dried up/ nowhere/ disappeared
There were few students who gave this type of response. However, there were still examples at all years.
This was not a common response, and by Year 7 very few students thought that the water had gone elsewhere as liquid water. An occasional student suggested that Tom might have taken Simon's water, or that Tom's might have leaked.
There were some examples of students using scientific terms incorrectly.
- dissolved by the sun (Year 4)
- dissolved into gas (Year 4)
- The sun melted it so it escaped (Year 4)
- Condensation melts back into water (Year 9)
a) iii)
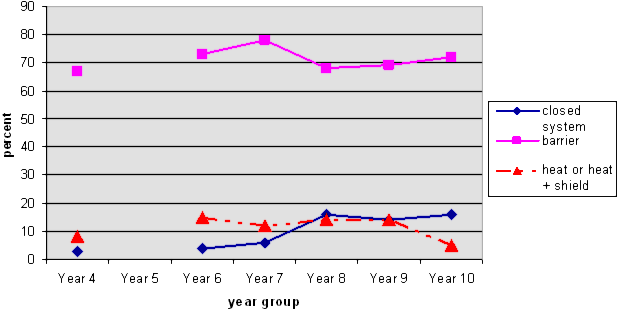
NOTE 1: The solid lines represent acceptable answers. The dotted lines represent misconceptions.
NOTE 2: There is no line linking the Y4 responses to the others because we did not include Y5 data.
This graph shows that at all years about 70% of students identified that the water couldn't get out because of the lid. This is known to students because of their observations in everyday situations.
Very few students described Tom's jar as a closed system, but there was a small increase between Years 6 and 8. Not all of these students mentioned a barrier, but would have been aware that there was one.
Students who mentioned heat know that there is a link between heat and evaporation, but they are still coming to grips with this idea. They may think that evaporation does not occur when it is cold (rather than a factor that speeds up evaporation), or they may be focusing on an irrelevant factor for the question (temperature rather than the barrier). Some thought that heat could not "get in" the covered jar, while others thought that the heat could not "get out". The graph suggests that this is more likely to happen at Years 6-8, after which most students realise it is irrelevant to this question.
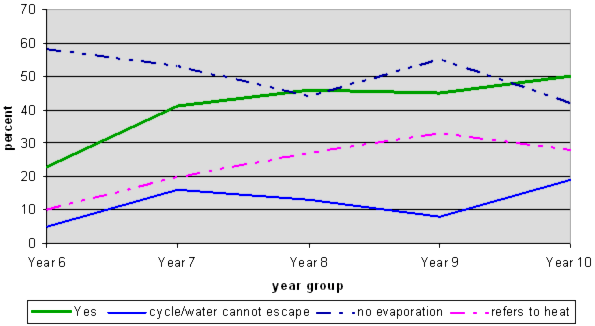
The thick green line (Yes) shows that at Year 6 most students thought that the water only evaporates in one jar. By Year 8 about half the students realise that evaporation takes place in both, but there is very little change from then to Year 10. The misconception that no evaporation was occurring in the closed jar was prevalent at all levels. These students are focusing on the water that is left (water is still there so it hasn't evaporated), and are not using particle theory to explain what happens to the water that has gone from the open jar. It is possible that, even though they may be able to use the word "evaporation" that they still think of the water as just disappearing.
Students found it difficult to justify their answer. Even at Year 10, only 20% gave an explanation in terms of the water vapour not being able to escape from the jar with the lid. Most who did, discussed the condensation forming under the lid as evidence.
Year 8 students were again more likely to refer to heat, but, unlike the last question, for this question a similar percentage continued to do so at Year 10.
Some students had some misconceptions about air.
There is no air in the jar with the lid on.
The water turned into air
Air combines with water to make more water.
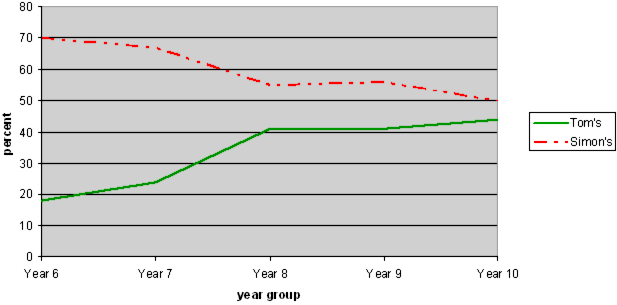
NOTE: The solid line represents the acceptable answer. The dotted line represents the incorrect answer.
About 70% of Year 6 students chose Simon's. There is quite a big reversal by Year 8. However, at Year 10 still about half the students chose Simon's. There was a close correlation between this choice, and thinking that evaporation only occurred in the open jar.
Question c) ii) is addressed in the section on Key competencies and Nature of science.

Exploring models potentially incorporates aspects of the Nature of science strand, and contributes to the development of the key competencies Using language, symbols, and texts and Thinking, as well as being an effective strategy for teaching science concepts.
Models are used by scientists to carry out investigations, to communicate their ideas, and as a thinking tool. Three models were incorporated into this task:
- the water cycle;
- the particle model; and
- the jar modeling the water cycle (a model of a model).
The trial students who completed this task generally had a poor understanding of models as representations. The next graph illustrates this.
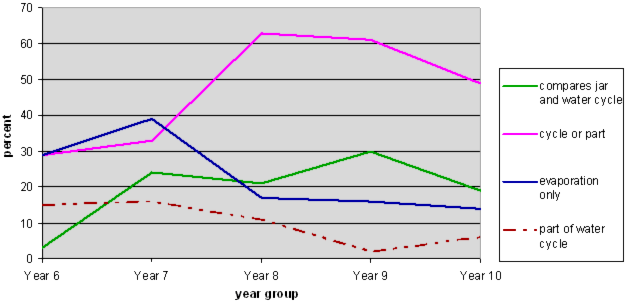
NOTE 1: The solid lines represent acceptable answers. The dotted lines represent misconceptions.
NOTE 2: Some students' responses included more than one category.
This question was analysed to see what elements of the jar model and the water cycle students included in their answers.
Identifies that the jar/ the jar and the Earth are closed systems
NOTE: This is not shown on the graph because so few students included this aspect in their responses.
More students' responses demonstrated that they understood that the jar was a closed system than the Earth is, by saying, for example, that the water could not escape from the jar and so the water kept cycling round. Only 3 students, all Year 10, referred to both the jar and the Earth's water cycle as being closed systems.
Makes specific mention of representations, e.g., the water in the jar represents the ocean
NOTE: This is not shown on the graph because so few students included this aspect in their responses.
Very few students were explicit that elements in the jar represented elements of the water cycle.
Makes comparisons between features of the jar model and the water cycle, e.g., the water falls down like rain
At Year 6 almost no students compared the jar and the water cycle model. By Year 8 this rose to 20%, but there was no further improvement by Year 10.
Describes a cycle (or part of a cycle), e.g., evaporation – condensation – evaporation
Discussed evaporation only
Younger students were more inclined than older students to focus on evaporation only. This was often linked to the inability to see that evaporation occurred in the closed jar. (91% of students across all years who discussed evaporation only, chose Simon's jar as modelling the water cycle.)
Students at Year 8 were more likely to describe a cycle or part of a cycle, but many focused on either the jar or the water cycle, without reference to the other.
One interesting misconception is also shown on the graph. The brown dotted line shows the percentage of students who envisaged the escaping vapour becoming part of the water cycle. All but one of these students also chose Simon's jar as modelling the water cycle, suggesting that their ability to interpret the model is hampered by their misconception that evaporation only took place in the open jar.
Next steps
Evaporation
Evaporation is a difficult concept for students. There are two things they need to understand before they can really create a mental model of evaporation:
- Water can be a gas (difficult because it is invisible); and
- Water is made up of tiny particles (i.e. the particle nature of matter).
To begin developing concepts of evaporation students need to be exposed to many examples of its occurrence in every day situations. Some examples of contexts students are likely to be familiar with are puddles drying up after rain, drying clothes, dishes drying on the bench, sweating, drying after a swim, and pots boiling dry. Discuss their observations and share their theories with others about what could be happening. Students will often retain their naïve explanations, e.g. "the sun sucks up the water", or "it leaked out" despite your best efforts. However, understanding a complex concept such as evaporation is a bit like doing a jigsaw puzzle. Once students have enough of the pieces they will begin to see how they fit together.
Explore condensation and evaporation together so it is easier to make sense of the disappearing/appearing water. Ask them to be creative, e.g. "Let's think of all the places the water may have gone", and also critical – "Is that very likely?" "Can we think of examples of when water just seems to appear from nowhere?" "What else have you noticed when this happens?" "Let's think of all the places it might have come from."
To help students focus on whether or not evaporation is occurring in both jars, set up the two jars as described in the question and encourage close observation of what is happening in each.
Role play provides a more concrete experience of an abstract idea. After observations, ask students to be the water particles in each jar. Older students can work in groups of three representing the chemical symbol for water. This will also reinforce the idea that the water molecule stays the same in each state – it is the movement and the spaces between molecules that changes.
Self-assessment
After observations are completed, ask students to revisit their first answer to Question b).
- Would they change their explanation?
- Did they notice anything that helped them rethink what they had written?
- What would they add or change now?
Ask students to share their ideas with a partner, small group, or the class.
- whether evaporation is occurring, and
- where the water has gone
that is important to focus on. However, in other circumstances, for example when we want to know how fast water may be evaporating, heat would be an important aspect to consider.
Put both jars in warmer and cooler positions, and compare the results. Does the water level still go down in the open jar when it is in a cold place? What do you notice about the closed jar? What is different between the jars in the cold and the warm place?
Developing scientific vocabulary
Introduce scientific terms. Using the word "evaporation" does not necessarily mean that students understand the process. However, using the correct scientific vocabulary cues students into wearing their "science hats", and contributes to developing a precise vocabulary to describe their experiences.
Interpreting models
Develop the understanding that models and analogies focus on part of the process or concept. Tom's jar can be regarded as a model for the condensation/evaporation cycle that occurs within the water cycle, but it doesn't address other elements such as freezing, melting, transpiration, respiration, etc.
Investigating the water cycle models a strategy for unpacking models. The same strategy could be applied to this model. When using models as analogies, once students have compared how they are like the target (in this case the water cycle), also explore where the model falls down. For example, although the jar and Earth are closed systems, the water in the jar is enclosed by glass (a physical barrier) whereas the Earth's atmospheric boundary is fixed by Earth's gravity.
- Ministry of Education (1998). Making better sense of the material world. Wellington: Learning Media.
- Ministry of Education (2001). Building Science Concepts, Book 15. Where's the water? Wellington: Learning Media.
- Ministry of Education (2003).Building Science Concepts, Book 31. Water and weather. Wellington: Learning Media.
ARB resources about evaporation:

