Researchers: Ally Bull, Rosemary Hipkins, Chris Joyce, Bill MacIntyre
Author: Chris Joyce (2008)
In 2007, the NZCER science curriculum team carried out a research project. Our questions were:
- What are New Zealand students' ideas about water and the water cycle?
- How do their ideas change as they progress through school?
What we did
We made up a "test" of six items. The questions were designed to investigate students' ideas about states of water and the water cycle. The focus of each question was informed by international research about students' understandings in this context. There was a mix of traditional questions, some less traditional questions, and further questions that unpacked students' thinking. Some of the items also investigated students' understanding of how science ideas are represented. Most of the questions were open questions, although there were some that were multiple-choice.
The questions are available on the Assessment Resource Banks. They are shown in Table 1. More detailed analyses for each question can be found in the Teachers' pages of each resource.
|
ARB resource |
Concepts explored |
Communicating in science |
|
Evaporation |
|
|
|
States of water |
|
|
|
Melting |
|
|
|
|
Evaporation |
Models |
|
The water cycle |
Models
|
|
|
The water cycle |
Diagrams |
Table 1 ARB resources derived from the investigation
The set of questions was answered by 655 students from Years 4-10 from 17 New Zealand schools. The sample is shown in Table 2.
|
|
Year 4 |
Year 5 |
Year 6 |
Year 7 |
Year 8 |
Year 9 |
Year 10 |
|
Student numbers |
99 |
15 |
115 |
51 |
128 |
64 |
183 |
Table 2 Numbers of students who trialled the material
- New Zealand students made a similar range of responses to those described in international research [arb: water cycle]
- Examples of the range of responses were evident at all years, except sometimes at Year 4. Few Year 4 students had a concept of particles.
- In many cases, but not all, a misconception was less frequently expressed by older students.
-
Many year 10 students still cannot explain changes of state. For example
- about a third did not say that water went into the air/atmosphere/room when it evaporated
- about half thought no evaporation took place in a closed jar
- about a third did not link an ice cube in a glass of water melting to the warmer temperature of the liquid water or to the air temperature
- about two-thirds did not identify that condensation forming on the outside of a glass of cold water came from the water vapour in the air.
-
Students at all levels had difficulty with what they cannot see. This includes
- water in its gaseous state
- processes such as evaporation and condensation
- particles and how they behave.
- Diagrams were often interpreted literally. They were not always seen as representations that focus on a small part of the reality.
- Students often had difficulty with explaining the relationship between a model and the elements of the water cycle. Their skill at expressing comparisons was poor.
- Scientific words such as melting, dissolving, and evaporating, were sometimes used to describe the wrong process. Other students used the word correctly but gave no indication of understanding the meaning. Sometimes other data from the test confirmed that they didn't understand the meaning.
Summaries of students' understandings as they progress through school
States of water
Science concept: Water can exist as a solid, liquid, or a gas within the range of Earth's normal temperatures.
Context of question: states of water in various places in the environment (MW6358)
- The easiest examples for students to identify the states were those they have observed, e.g., the sea, snow, rain, and hail.
- Between two-fifths and three-fifths of Year 4 students did not identify the correct state for these easier examples. One factor may have been that they were unfamiliar with the scientific language, e.g., state, liquid, solid, gas.
- Contexts that students had not experienced were more difficult. Glaciers, inside us, and inside a plant are examples. Older students were more likely to be able to describe the state of these.
- Air in the room was a difficult question for younger students. By Year 8, however, about four-fifths were aware that water can be a gas.
- Most students at all years identified that clouds and fog were predominately a gas. The invisibility or transparency of water vapour is a barrier to them understanding that it is there even when we cannot see it.

Implications for learning
-
Start with examples that students can or have experienced. Observation is an important scientific skill to develop in this context. Next, explore contexts that
- are familiar but students may not recognise as including water (our breath, water in plants)
- are less familiar (glaciers, polar ice, artesian water).
- Introduce water vapour (water in its gaseous state) after solids and liquids are understood.
- Look for ways to make the invisible "visible", e.g. role play, imagining. Encourage students to share and debate their ideas.
- Introduce science vocabulary once students recognise and attempt to describe in their own words the process.
Melting
Science concept: The warmer temperature of the medium (e.g., water, air) an ice cube is sitting in causes melting
Context of question: ice in a glass of water (PE7577)
ice melting
Most students at all years can name and describe the process of melting.
However, the ability to explain what causes it to happen is poor.

Implications for learning
- Students need opportunities to notice and talk about the conditions that cause melting. The role of temperature should be included in their explanations.
- From years 7 and 8 students should be starting to consider energy and particle theory in their explanations of change of state.
Evaporating
Science concept: Water from washing on the line goes into the air/ sky/ clouds/ atmosphere/ as it dries.
Context of question: washing hanging on a clothes line, Disappearing water (MW6302)
Percentage of students understanding that water from washing goes into the air as the washing dries
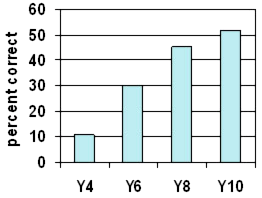
The graph shows a steady progression in understanding this concept. However, at Year 10 about half still did not indicate where the water had gone. From Years 7-10 over half the students used the term "evaporated". However, many of these did not explain the term further. They may or may not have known the answer to the question.

Implications for learning
- Check that students know the meaning of technical science words.
- Water vapour is difficult to envisage because it is invisible. It is often confused with steam or clouds. Provide opportunities to debate their ideas about what is happening in a variety of experiences.
From years 7 and 8 students should be starting to consider energy and particle theory in their explanations of change of state.
Science concept: Evaporation will occur in both a closed jar and an open jar. The evaporated water cannot escape from the jar with a lid.
Context of question: water in a closed jar and an open jar, Where did the water go (MW6356)?
Percentage of students understanding that evaporation
occurs in both open and closed jars
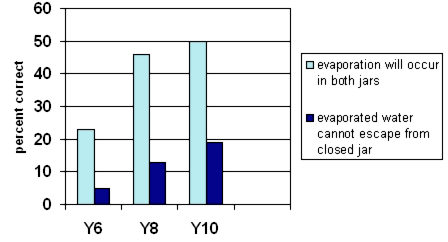
By Year 10 about half the trial students knew that evaporation took place in both jars. Less than a fifth explained their answer by linking to the evaporated water not being able to escape from the jar with the lid. (Year 4 students were not asked this question.)

Implications for learning
A misconception was that evaporation occurred only in the open jar. The reason given was that the water level stayed the same in the closed jar. Many students still hold some notion that the water disappears or goes somewhere else when it evaporates.
Close observation of the condensation forming under the lid of the closed jar helps students to understand that:
- water does evaporates in the jar
- evidence of this is the water forming (condensing) underneath the lid
- like in the "water cycle", the water continually changes between its liquid and gaseous state
- like water on Earth, the water is contained (it cannot escape).
Science concept: Substances dissolved in water are left behind when water evaporates.
Context of question: explaining why rain is not salty, The water cycle (PE7553)
understanding that dissolved
substances are left behind when
water evaporates
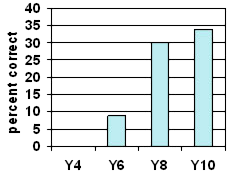
No Year 4 trial students demonstrated a complete understanding of this concept. This is not unexpected. However, neither did about two-thirds of the Year 10 students.

Implications for learning
- There were 2 main misconceptions expressed by the trial students
- Salt is too "heavy" to evaporate.
- Rain water doesn't originate from ocean water.
- Students at all levels can investigate evaporation of a solution. Growing crystals is one interesting context to do this.
- At Years 7 and 8 students can explore the energy requirements for evaporation to take place. They can investigate the boiling points of a range of substances.
Science concept: The water that forms on the outside of a glass of cold water comes from the air.
Context of question: ice in a glass of water (PE7577)
Percentage of students understanding that condensed
water comes from the air
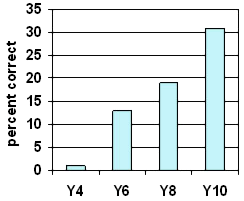

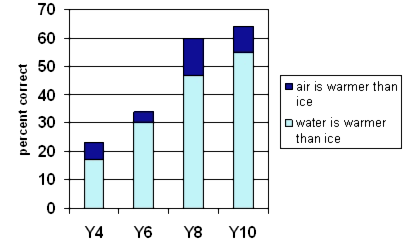
Almost no Year 4 students could answer this question. At Year 10 only about a third identified that the water came from the air.
The misconception that the water comes through the glass was common at all levels.
At Years 7 and 8, students were most likely to mention cold temperatures. In some other responses coldness was implied. Some had the misconception that coldness goes through the glass to produce water.
Older students were most likely to write, "It is condensation" without any further explanation. They may or may not have known that the condensation came from the air. Very few Year 4 students used the term "condensation".
Implications for learning
- Check that students know the meaning of technical science words they use.
- Provide opportunities for observing examples of condensation forming.
- Provide opportunities for students to debate their ideas about what is happening.
- From years 7 and 8 students should be starting to consider energy and particle theory in their explanations of change of state.
The water cycle
Science concepts: Refer to key on graph.
Context of question: comparing a model of a water cycle with the real water cycle, Investigating the water cycle (PE7526)
The graph reports on students' understanding about the real water cycle.
Percentage of students understanding
concepts about the water cycle
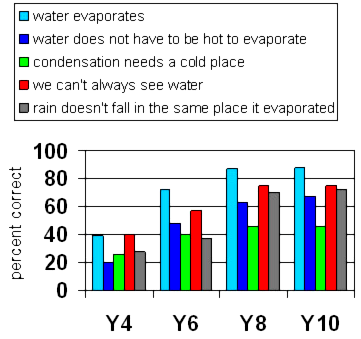
Students progressively understand elements of the water cycle. However, there is little progress between Years 8 and 10. At Year 10 a significant number still hold misconceptions.

Implications for learning
- Revisit the water cycle throughout students' schooling, so understandings are built on.
- Condensation seems to be an area of difficulty. The question did not give students the option of relative coolness, which may have caused some difficulty. For example, the bathroom wall where they may see condensation forming may not be cold. It is, though, cooler than the air.
Models
Communicating in science: A model is a representation of something. Elements of a model can be compared to elements of the real thing.
Context of question: the water cycle, Where did the water go (MW6356)?
model to water cycle
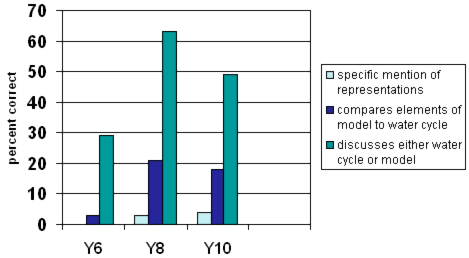
Students at all years were most likely to discuss either the model or the real water cycle, without drawing comparisons.

Implications for learning
- Consciously scaffold students to compare elements of a model with the real thing.
- Examine where the model differs from the real thing.
-
Develop vocabulary that enables students to compare, e.g.,
- The drops falling from the lid are like the rain.
- The water represents the ocean.
- The jar is different from the Earth's water cycle because there are no living things in it.
Diagrams
Communicating in science:
A diagram is a representation of something. Arrows are one convention used in diagrams. In science, arrows have particular meanings which are context specific. We have to interpret what the arrows mean when we are reading a diagram. In a water cycle diagram the arrows represent a change of state and/or change of location.
Context of question: Students had to correctly place the arrows on an unfamiliar water cycle diagram, The water cycle (PE7553)
This question was very difficult for students at all years. Only 13 out of 655 students got all arrows correct. Those who did were in Years 5, 6, 8, 9, and 10.

Implications for learning
Rather than just reproducing diagrams, assist students to
- interpret what the diagram is showing
- recognise the conventions used by the writer and what they show in this context
- think about what is not shown and why
- understand the relationships between parts of the diagram
- become aware of what cannot be read literally (e.g., the arrows do not point downwards to show that the rain falls downwards).

